The development of a supply chain data management system should ideally include stakeholders from business entities supporting drug manufacturing operations. Typically, representation from finance, facilities, manufacturing, operations and quality systems is required to completely map out the supply chain management work streams. Each entity will dene critical data capture and reporting needs related to their respective functional areas and help shape the structure and utilization of the data collection, analysis and reporting tools. The integration of supply chain management data with other enterprise data enhances the coordination of the activities of various stakeholders in the supply chain. Careful forecasting of demand, logistics, costs and time for implementation are typical drivers of decision making in this area. The involvement of 3rd party logistics providers (3PL) in the supply chain will add another level of complexity both in the design of the data capture and management system as well as integration across stakeholder platforms. Figure 1 shows critical elements and information flow between various stakeholders of a typical supply chain data ecosystem within an organization.
CALL US TODAY · (800) 959-9570
Blog Layout
Developing and implementing a supply chain management system for cellular therapy programs
David L DiGiusto, Rakib Ouro-Djobo & Uzair Rajput • April 16, 2020
RAW AND STARTING MATERIALS: TROUBLESHOOTING SUPPLY, MANAGEMENT AND OPTIMIZATION
The past few years have seen explosive growth in the development of cell and gene therapy drug candidates for oncology and genetic diseases. There are currently 17 approved Cell and Gene therapy products as listed by the US FDA (FDA Approved Cellular and Gene Therapy Products). Current and pending approvals of immune cell products in particular (CAR-T, Dendritic, hematopoietic stem cells) have driven substantial demand for increased cell manufacturing technologies and capacity. Additional advancements in iPSC-derived cell-based therapeutics (nerve, bone, skin, cartilage, bladder, cardiac, liver tissue repair and regeneration) are also driving the development of cell manufacturing technologies. The combined growth and demand for increased production capacity has led directly to an increased need for raw materials, facilities and services. The raw material and product supply chain is a critical element of a manufacturing program for cell therapies. The development and implementation of a robust supply chain management system (SCMS) is required for the successful development of any cell therapy platform. An SCMS is the collection of policies, procedures and tools used by manufacturers to dene, control and document the flow of materials into and out of manufacturing campaigns. The main purpose of SCMS is to ensure the provision of an uninterrupted supply of clinical materials that meets all regulatory requirements as per the Code of Federal Regulations 21CFR§210,211 Good Manufacturing Practices (GMP). The SCMS must include identification and specifications for raw materials as well as sourcing and qualification of all providers of raw materials and services. It, also, must provide for documentation on procurement, shipping, holding, testing and product distribution with traceability throughout the process and tracking of process intermediates and final drug product. The system must reliably capture and report out supply chain data in a manner that supports continuous cell manufacturing and future process planning and optimization (e.g., materials mass balance). In this piece we highlight the major components of a successful SCMS and give examples of approaches for supply chain management that help to facilitate control and compliance, reduce risk and ensure the continuity of clinical materials production.
DOI: 10.18609/cgti.2020.042
Citation: Cell & Gene Therapy Insights 2020; 6(2), 325–337
Supply chain management systems
Source materials development
Tools and techniques developed for pharmaceutical supply chain management can be applied to cell-based therapeutics for the management of much of the raw materials supply chain. However, cell therapies are ‘living drugs’ which present a few unique supply chain challenges. For example, cell-based therapy products are often manufactured using primary tissue isolates either from the intended patient (autologous) [1–3] or a third party (allogeneic) source [4–6]. Individual primary tissue isolates (e.g., apheresis products) have significant differences in composition and quality and have been shown to be the greatest source of variability in cell manufacturing process with impact on product quality and yield [1,7]. Additionally, autologous cell therapy source materials must be carefully tracked from collection, through manufacturing and back to the correct patient at infusion (so-called ‘vein to vein’). Patient-specific supply chain tracking is unique to cell therapies and not addressed in traditional pharmaceutical supply chain models. Finally, certain cell therapy products are infused immediately following manufacturing (without cryopreservation) and thus have very limited ‘shelf life’. These products cannot be ‘held in inventory’ prior to use and require rapid controlled disposition and distribution. Thus, unlike traditional pharmaceutical products, cell therapy supply chain management will include real time tracking and distribution that ensures product integrity and delivery to the intended recipient. Collectively, these challenges must be addressed for each cell therapy product through development of critical quality attributes that ensure the suitability of products for clinical use.
Most cell therapy drug candidates are first identified in an academic laboratory or other research setting and typically employ research grade reagents and supplies. All raw and source materials used in the manufacturing of drugs (cells) intended for clinical use must meet specific quality and safety standards that often exceed those of research reagents. Therefore, a first step in cell product development is the identification of suitable sources of required raw materials and qualified vendors of those materials. A bill of materials (BOM) is a description of all raw materials, supplies, vendors, quality specifications and testing schedules and is used to catalogue the essential components of a manufacturing process. The BOM includes all the information collected for specific materials and vendors and is used as a ‘procurement shopping list’.
The initial focus during BOM development is to identify and dene the attributes of a safe and reliable source of raw materials (Raw Materials Specifications) and establish GMP compliance in a ‘clinical trial phase-specific’ fashion throughout product development and clinical testing. A more comprehensive supply list may augment the BOM and encompasses many programs and departmental supply requirements that dene the total procurement requirements in support of multiple programs (environmental testing, cleaning, release testing, standards). The BOM and supply lists are reviewed and approved by the Manufacturing and Quality units to ensure compliance with manufacturing requirements and pre-established quality standards. The BOM is typically used by a supply chain (operations) group to assure material availability and manage required interactions with other planning and finance groups.
As described above, limited control over the quality of a primary tissue harvest makes creating raw material specifications challenging. The current best practice for setting raw material specifications includes the use of healthy donor material during product development and performing confirmatory studies on actual patient products where available [8]. Primary cells as raw materials will typically have a wide acceptance range for raw materials specifications to accommodate the inherent variability of the patient population. Variability can be reduced, for example, by using starting materials from a single or limited source of qualified (allogeneic) healthy donors (blood, biopsy) or decidual tissue (cord blood) and creating banks of cells expanded in vitro with retention of desired biological properties (allogeneic CAR-T and MSC References). Source materials may include Master and Working Cell Banks derived from a source tissue as well as cryopreserved cell processing intermediates that must be tracked throughout manufacturing and distribution. Process centric risk assessments drive the extent to which a raw material supply is tested and established to be safe and reliable for manufacturing of a cell product. If research reagents are replaced with more ‘qualified’ sources (e.g., GMP grade), comparability of biological activity must be verified. Each raw material will require the use of qualified analytical tools for characterization and stability measurements. In addition to basic cell characterization (counts, viability, identity) these third-party materials must be thoroughly characterized for donor suitability (21CFR§1271) prior to use and will always require some form of immune protection when administered.
Non-cell based raw materials may also come from human sources (serum, plasma, platelet lysate) and are subject to strict quality standards associated with blood and tissue donations. While many cell collection (apheresis) and processing steps (platelet collection) can be harmonized using standard operating procedures and similar equipment, lot to lot variability is significant. Periodic supply interruptions (low donor frequency) or non-conformances (infected or otherwise disqualified donations) can threaten the continuity of raw materials. Other raw materials for cell manufacturing include cell culture media and supplements, cell processing buffers and enzymes, growth factors, nucleic acids, viral and non-viral vectors and small molecules.
As products show promise in pilot clinical studies and move towards pivotal trials, supply chain activities turn towards ensuring full compliance with regulatory standards. It is typically at this stage where extensive raw material testing and qualification (e.g., stability) programs are implemented. In many cases, critical raw materials come from vendors who only provide research grade reagents, do not have the ability to produce lots at clinical or commercial scale or are sole source providers. Assay development may be required to qualify raw materials beyond what is offered by the vendor. Few of the required assays are compendial in nature and will often require significant development using knowledge of what raw material attributes determine suitability for intended use. Each of these represents a risk to supply chain reliability and continuity and should be considered as early as possible in supply chain development (See also Procurement, below). The knowledge gained during materials selection will be critical to guide subsequent scale-up and product comparability studies.
Procurement
Procurement is a set of business processes encompassing planning, purchasing, inventory control, receiving, and receiving inspection. Materials procurement must be designed to support manufacturing projections for cell products using a systematic approach. A systematic approach first consists of breaking down the planning process into steps to eventually mitigate risks such as backorders, lot-to-lot variability for biologics and small molecules in addition to shortage of ancillary materials amid manufacturing productions. Second, as described above, capture of metadata from early lots of material used in each manufacturing production will help establish a knowledge base and support trending analysis for supply chain logistics. Metadata includes information on pricing, lead time and availability, reagent grade, as well as vendors’production capacity and history of support for scheduled cell manufacturing productions.
Identication of the most critical components of a supply chain is part of a basic risk analysis. Materials with long lead times as well as materials from sole source vendors are conventionally considered critical, therefore robust controls and monitoring should be implemented. Such items are usually highly priced as well which makes it even more important to exert appropriate controls to eventually lower the Cost of Goods Sold (COGS). Additionally, critical but ‘non-GMP grade’ materials may be the only source available in early stage clinical studies. They constitute a higher quality risk due to less stringent manufacturing control and documentation and thus a lower degree of compliance with applicable regulatory requirements. Ranking materials based on criticality is part of risk assessment. More critical items should be secured with greater assurance (larger inventory, secondary vendor, supply agreements) and will likely be procured to minimally support several lot-production runs at once. Purchases are made with a clear understanding of lead time and time for lot testing and release as required. At all times procurement must consider component expiration dates to meet demand. Long range planning creates the opportunity to reduce COGS by negotiating pricing with vendors, who often provide price incentives as part of a committed bulk order. Finally, the collected data can now be incorporated into the raw materials forecasting to meet manufacturing demand planning which in turn informs procurement strategies. It is important for the supply chain department to understand their manufacturing processes and stages at which critical materials are required and used during the manufacturing production to prioritize the procurement and storage of those materials. During the planning phase, it is also important to identify materials that are readily available and procured using the just in time approach.
Procurement should be tailored to clinical material production cycles in a way that ensures the availability of the required components at the projected time of manufacturing. Long lead time items can create a level of uncertainty that requires special considerations to ensure order fulfillment. A supply of 6- to 12-month of projected inventory is not uncommon. Lot expiration limits storage lengths and order fulfillment timing can result in just in time deliveries of many materials. Annual estimates for materials need as well as annual financial forecasting is derived from procurement records and subsequently the source of information used to project cost of goods.
Once manufacturing has been initiated, the management of the drug substance and final product from the manufacturing site, throughout storage and ultimately to the clinical center for administration is also a critical aspect of the supply chain management. As described above, many cell therapy products require ‘vein to vein’ traceability and may have short ‘shelf lives’. Therefore, clear definitions of transport procedures, practices and limits are crucial. The development of a SCMS for each ‘living medicine’ must include development and qualification of methods for transport and delivery of the final product to the clinical center for administration (see Shipping, below). As a product works its way towards commercialization, a Sales and Operations Plan (S&OP) is developed that describes the intended procedures and for production and distribution of the cell therapy product in order to achieve low cost and maintain a reliable procurement program. Once the S&OP process is well understood it becomes simple to forecast the consumption of materials, which in turn helps establish appropriate levels of inventory at cell processing facility as well as throughout the organization’s complete supply chain.
Vendors
Delivering cell and gene therapies is an expensive and highly complex process and strong client-vendor relationships are critical to success. Cell therapy companies benefit from finding vendors who are willing to collaborate extensively and are capable of leveraging other relationships to bring value to the organization. The interactions with the supplier should be ongoing and close attention should be paid on the attentiveness of the supplier. These interactions should occur at all levels of each organization and be supported by the senior management of each party. For example, quality audit, overall site evaluation visits, meetings at cell therapy company offices, etc. These interactions should be viewed as opportunities to cultivate strong relationship with the supply chain company and in the long run will pay off in terms of overall value the supplier brings to the table.
Prioritizing vendors based on the criticality of their products to the process and general availability is also important. Raw materials that are available from multiple qualified sources are important but should be given second priority for review compared to those products obtained from sole source providers. Procurement of specialized or unique materials from single vendors in the industry is perhaps one of the biggest supply chain risks to many operations. Due diligence for vendors often includes, but is not limited to, evaluation of vendor production capacity, raw material grades available (Research, GMP, USP, licensed drug), time for manufacturing, company financial health, in addition to their location in respect to the manufacturing site. When selecting vendors, it is also important to identify vendors who are flexible to work with manufacturer as they are still in the development phase of sourcing new materials. Vendors who are flexible will usual be willing to work with clients to establish a long-term relationship that will support product growth overtime.
A significant contributor to the success of supply chain development is the establishment of strong relationships and well-defined supply and quality agreements between manufacturers and clients. Supply agreements will cover companies’ raw materials needs planned throughout the year, in addition to any applicable discount vendors may offer during that period. Quality agreements are equality important and should be in place alongside supply agreements. They contain terms that cover compliance and assurance of quality in production of the raw materials. A quality agreement will ensure that any changes to the manufacturing or sourcing of a raw material or component of a reagent will not be done without notication and/or review by the drug manufacturer (vendor’s client). Additional examples of critical supply chain and quality agreement components are given below in Box 1.
It is often difcult for cell therapy manufacturers to determine qualication requirement for a raw material. At this stage in the industry, no single material-grade, manufacturing standard or any standardization of other compliance claims, such as ancillary-grade, clinical-grade, GMP-grade, and animal-component-free exists. Although some regulatory hurdles have been reduced within the last few years, it remains an expensive endeavor to develop and process cell therapy products. With constraints on time and resources, it is essential to find ways to reduce waste. This is where the benets of the utilization of LEAN methodologies can come into play [9]. Supply chain partners who thoroughly understand LEAN and have implemented LEAN systems can quickly become the ‘supplier of choice’ for most companies. Such LEAN suppliers often bring value by streamlining the supply chain while continuously looking to eliminate waste from the entire value stream of cell therapy.
LEAN Thinking in procurement is essential. Due to extensive growth within the cell therapy industry, many labs and startup companies may not have the resources to perform all business processes with individual suppliers as required by their Quality Management System. Therefore, companies with limited bandwidth should attempt to minimize the number of suppliers, placing the burden of compliance on their suppliers. In this way, it may be benecial to nd a third-party service company who can act as a procurement agent, managing the value stream of many components, compounding or compiling many units to one and/or providing the individual components, when necessary, to the cell therapy company. With only one company to manage, and fewer products to inventory, the cell therapy company reduces the need for further bandwidth. Barcoding incoming supplies would be another aspect of LEAN techniques. With the massive number of items needed to process a batch of cell products, reading individual components and transcribing the traceability information even into a computer can be time consuming and should avoided when possible.
Receiving inspection
In order to ensure that a supplier complies with the requirements of the Raw Material Specication (RMS) for cell therapy raw materials, it is paramount that specications are well understood throughout the value stream. Ideally speaking, every component, ranging from pipette tubes to sera and reagents, should have well dened specication sheets, communicated to the supplier, and verified through signature process to ensure that there are no mistakes. An additional benet of using specication sheets is that they allow the ‘warehousing’ function at cell therapy companies to evaluate the supplies upon receipt in their receiving inspection processes. The Receiving Inspection Process is an important element within an established Quality Management Systems for Cell Therapy companies. This safeguards regulatory needs as well as administering the highest standards of patient safety, all of which require assurances that the material is appropriate for use and has strong traceability.
All products arriving at a manufacturing site are typically inspected for container integrity, temperature, identity and alignment with procurement records. Materials are held, documents certifying materials (certificates of analysis, origin, conformance) are collected and entered into a data capture and management system (see below). Sampling of lots of materials for release testing may also be performed at this time. Materials not meeting any of the technical, procurement or shipping requirements will be rejected and must be held in a manner to prevent mix-ups with accepted or in process components. Receiving inspections processes are the first line of control of materials in the value stream and thus are of critical importance to be managed against a well-dened operating plan.
Inventory management
Inventory management is critical to the success of well-functioning cell therapy supply chain. Many organizations fail to understand the importance of inventory management, treating it as a second level priority activity. Inventory Management services can be implemented to ensure proper materials forecasting, first in first out (FIFO) consumption of goods and just in time reordering of supplies as they are consumed. The inventory management system must account for any expiring components that might jeopardize the quality and continuity of the supply. This matter of expiration is one of the issues that requires established business reviews between supplier and cell therapy companies to ensure that monthly usage is tracked, and inventory levels are adjusted accordingly. Eliminating the waste of unused but expired goods is also critical step in order to minimize the overall cost of goods sold (COGS).
At the terminal end of the supply chain, the focus on health and safety are directed towards patients’ safety. All products must be managed throughout production and testing and during shipping to the patient to prevent mix-ups resulting in the delivery of the wrong product or dose to the wrong patient. Bar coding systems are often used to label and track products and product labeling standards such as ISBT 128 have been developed to address these requirements. See also Shipping, below.
It is worth mentioning here that supply chain management must also include a plan for the impact of raw materials on the environment; or on the health of the manufacturing staff and end users (patients). Safety measures are typically directed towards storage of the raw materials in the proper temperature and space and in the correct packaging to prevent spills. Additionally, testing may be required for animal derived sources of materials (e.g., serum, albumin, transferrin, platelet lysates) for the presence of adventitious (infectious) agents and acute toxicity for concentrated forms of other reagents (e.g., retinoic acid, solvents, concentrated acids and bases used to pH media and buffers). A complete and thorough review of the BOM for any product should be performed with environmental health and safety (EHS) specialist to ensure the proper storage and handling of all materials.
Cell therapy manufacturing requires the use of a considerable number of biologics, small molecules, growth factors and a variety of types and sizes of custom plastics with attendant physical and chemical hazards and risks. Final products are well tested for safety prior to use in humans. However, it is extremely important to discuss the impact of exposure to raw and waste materials on the health and safety of at-risk staff (shipping, handling, testing and manufacturing staff) as well as impact of material use and disposal on the environment. These discussions should happen during planning and forecasting with an internal health and safety ofcer or department prior to initiating their sourcing. Doing so, a proper waste management plan will be crafted in accordance with local cities rules and regulations to potentially mitigate public health risks in cities where media disposal rules and regulations are minimal and have yet to catch up with these new trends.
At the time of writing of this article the world is coping with the C0VID-19 viral pandemic. Lives are impacted in many ways but one of the more subtle disruptions is the impact on supply chains throughout a wide variety of industries and commercial sales. These events emphasize that disaster planning and recovery should be a consideration for supply chain continuity in cell therapy.
Shipping
The transportation of raw materials to manufacturing sites and products from manufacturing sites to clinics requires demonstrable control over the transportation process and documentation of the history of shipped material. Working with vendors and transportation providers who understand and can support these shipping requirements is critical. Tracking shipment location and temperature are critical parameters part of the value stream of cell therapy. Delays in transportation due to weather, customs, agricultural inspection and other reasons can adversely affect the quality or availability of critical supplies. The shipment of raw materials and drug products with temperature sensitivity (-20 C, 4 C) must be recorded to ensure maintenance of material quality.
Several mechanisms exist to monitor and report on shipping conditions and even real time location of products. For example, data recorders (e.g., TempTale ) can be placed inside the shippers to record the temperatures which can be transmitted to the virtual (cloud-based) storage locations in real time or downloaded at end of shipping cycle to ensure that the cell products did not experience out of specification temperatures. GPS tracking systems such as CryoPortal /Smartpak II system and cloud-based data recording (e.g., SenseAnywhere ) track samples from pickup through delivery to the end user and provide real time monitoring of location with 24/7/365 traceability.
Validation of shipping systems is also required to ensure compliance with industrial standards such as Good Distribution Practices (GDP). Guidelines for shipping and handling of drug products or active pharmaceutical ingredients (API) have been developed in the EU (Article 1(33) of Directive 2001/83/EC) and by WHO (World Health Organization – WHO Technical Report Series, No. 957, 2010) and are currently under development in the US. must include ability of the shipping system to maintain temperature and protect the cell therapy product physically. In cases where validation must be performed, some form of verification techniques must be employed. To this end, several other standards are also available that can be followed, e.g., ISTA and ASTM.
Electronic data capture & management
Supply chain data capture, management and analysis are critical activities designed to ensure operational efciencies and regulatory compliance during product manufacturing and distribution. Many small companies and academic institutions approach this task initially with spreadsheets and text documents but quickly realize the limits of these stand-alone data sources. A more reliable approach is to develop (or purchase) software that can integrate the planning, procurement, management and nances of supply chain activities. Enterprise Resource Planning (ERP), Manufacturing Execution Systems (MES) and Inventory Control software are widely available (e.g., TrackCel) but the integration of these packages with each other can be challenging, somewhat time consuming and expensive. Inter-platform data transfer presents challenges in both dening relationships between disparate data sets and verifying the integrity of the data upon transfer between systems. Achieving a robust system for inter-platform data management requires signicant time and investment in ‘systems engineering’ and infrastructure when licenses or technologies to allow systems to interface are not always available from the software vendors.
An alternative approach is to build a system on a platform technology that can be used to integrate disparate enterprise system and product-specic materials management data. An example would be to implement a congurable Laboratory Information Management System (LIMS) that is designed to accommodate supply chain but also integrates with other nancial and facilities planning software. We have previously described the development and implantation of such a system to aid in the operations of an academic cell therapy laboratory [10]. The time and effort required for implementing each system will vary and it is prudent to begin the assessment of supply chain software needs through a gap analysis in which all of the above parameters can be mapped out into a process flow diagram to determine the flow of materials, data collection points, interrelations between data sets and expected reporting requirements. Production demand can be overlaid on the process flow diagrams to determine cadence of ordering and supply consumption and allflow for establishing materials management efciencies using Lean manufacturing principles. Creating a supply chain data management plan from such an exercise will enhance the likelihood of a successful system implementation and establish responsibilities, timelines and cost of implementation.
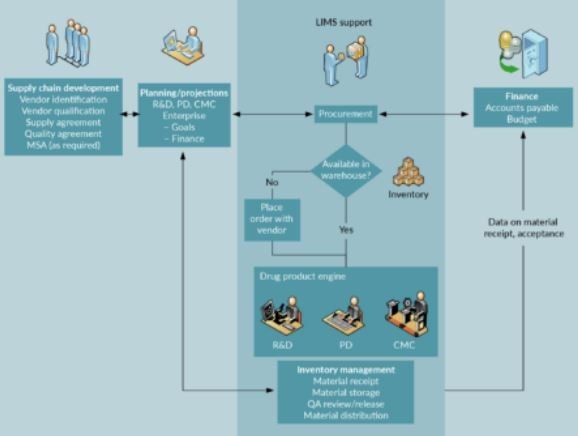
Quality Systems personnel can control the BOM and supplier lists, implement changes and add or eliminate items in a regulated fashion. Procurement can then place orders based on this controlled materials sourcing dataset which will substantially reduce the risk of use of non-qualied materials or vendors. A qualied vendor and material list also allow procurement to mitigate backorders or other order fullment delays by having pre-approved alternatives on record. Linking enterprise systems for procurement (ERP) to materials receipt and management allows for traceability of orders and facilitates payments of materials received and accepted. MES or LIMS systems can (and should) be designed to support (optimize) warehouse utilization and quarantine/release logistics once inventory is received and materials are assigned to ambient or cold chain storage. The information related to the BOM and specications can also be used to plan, procure, receive, store, qualify and distribute materials. Trending of material use rates, failure rates, delivery delays and other supply chain metadata will support continuous process improvement and operational efciencies in the manufacturing plant.
Throughout production, the assignment of BOM items to each campaign is recorded to serve as the record of raw materials usage in each batch. The data is also used to determine inventory levels in real time and to facilitate product reporting requirements in the event of a raw material recall. The information on usage will also be used to guide lean manufacturing practices and just in time supply procurement. During the creation of the drug product, manufacturing process intermediates and drug substance are critical materials in the supply chain must be tracked as would any other production material. Analytical and storage data must be captured as does signicant amounts of product metadata (manufacturing date, batch release, storage conditions and lot disposition) to facilitate product distribution and recall.
Shipping
FDA expects that all data collected as part of a batch record (including materials) be complete, consistent and accurate (21 CFR§211). Records keeping traditionally has been achieved using paper records but the movement towards electronic records is becoming more common. Electronic record keeping and signature requirements are specied in 21 CFR§11 and in an FDA guidance document (Data Integrity and Compliance With Drug cGMP Questions and Answers – Guidance for Industry). FDA recommends that rms employing electronic records should implement meaningful and effective strategies to manage their data integrity risks based on their process understanding and knowledge management of technologies and business models (see also, ICH guidance for industry Q9 Quality Risk Management). There are standard practices and procedures that can be undertaken to ensure data integrity. Controls should be in place to ensure that the data is complete, entered at the time of performance and protected from adulteration using secure access practices. Any required changes to data records should be performed in a documented fashion with Quality Systems oversite, review and control. The safety and integrity of the data should be ensured through qualied backup procedures with periodic audits to ensure the integrity and completeness of the backup. Where available, original paper records can also be used to verify an electronic version of the same information. Mechanisms such as controlled login and traceability of entries and changes also help to ensure the integrity of electronic data sets. Systems should also be in place to detect omissions and other errors if and when they occur (e.g., incomplete records, lack of units for measurements, etc.). Metadata (that is information that puts all data into context) is also part of electronic records and must be recorded and maintained with the same level of integrity as raw data. All data must be verified through a secure (non-corruptible) audit trail containing information on times, dates and other information that allows for a complete reconstruction of events recorded in the data set.
Data collected and stored in an electronic system should be backed up using a reliable method that represents a ‘true copy’ of the original record. Backups can be in electronic or original paper format but must be verified to be accurate and complete. The design of systems and software used to store supply chain and production data are typically validated by the vendors at the time of creation but implementation of each system for each installation and process will also require some level of validation. These services should be planned ahead of the implementation and use of data capture and storage systems.
Conclusions
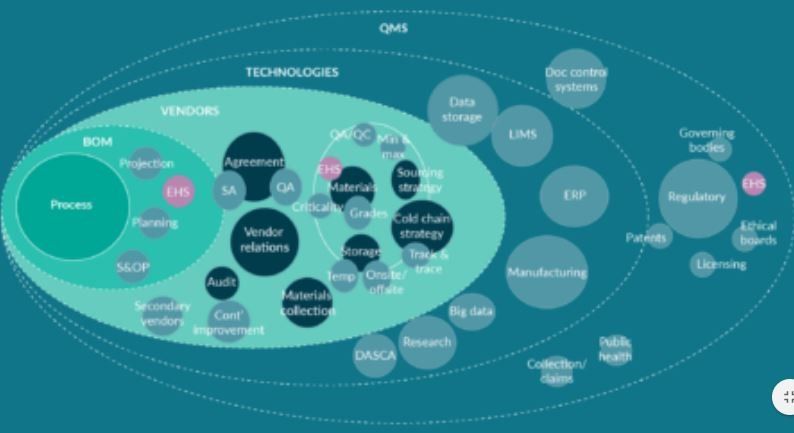
The development of cell therapy supply chain management system is both complex and essential to the successful development of a cell therapy product. Demonstrable specication of and control over all source materials from the time collection/procurement, throughout manufacturing, shipping and delivery of nal formulated cell product to the patient is critical for the safe and effective use of these products. The supply chain is built around a manufacturing process, sourced against a BOM using qualied vendors, procured and tracked using ERP and other data management technologies and overseen by the QMS policies and procedures of the organization. Figure 2 outlines the universe of disciplines, technologies and stakeholders involved in supply chain development.
The take home points of this article can be summarized as follows:
- The development of a supply chain management system is a complex, time consuming task that is essential to the success of a cell-based drug development program;
- Cell therapy products are living drugs and have unique supply chain requirements both for raw materials as well as final product;
- Supply chain needs will largely be driven by production and quality requirements but contributions from all disciplines of the company is required to develop a cohesive supply chain system that serves all compliance and corporate operational needs;
- The development of a supply chain management system is a complicated process and should be initiated early in the product lifecycle;
- Sufcient resources and systems should be identied to ensure the continuous availability of safe and effective clinical materials
Share
Tweet
Share
Mail
